Virginia Medicaid Preferred Drug List (PDL) Program Changes including Hepatitis C Drug Changes Effective January 1, 2017 and Drug Utilization Review (DUR) Board Approved Drug Service Authorization (SA)
 Download PDF
Download PDF
The purpose of this memorandum is to inform providers about changes to Virginia Medicaid’s Fee-for Service Preferred Drug List (PDL) Program that will be effective on January 1, 2017 and new drug service authorization (SA) requirements approved by DMAS’ DUR Board.
DMAS Drug Utilization Review Board Activities
The DMAS Drug Utilization Review Board (DUR Board) met on November 10, 2016 and recommended that DMAS require prescribing providers to submit a Service Authorization (SA) for Venclexta™ (venetoclax) based on FDA approved labeling.
Preferred Drug List (PDL) Updates – Effective January 1, 2017
The PDL is a list of preferred drugs, by select therapeutic class, for which the Medicaid Fee-forService program allows payment without requiring service authorization (SA). In designated classes, drug products classified as non-preferred will be subject to SA. In some instances, other additional clinical criteria may apply to a respective drug class which could result in the need for a SA.
The PDL program aims to provide clinically effective and safe drugs to its members in a cost-effective manner. Your continued compliance and support of this program is critical to its success. The PDL applies to the Medicaid, FAMIS, and FAMIS Plus Fee-for-Service populations. The Virginia Medicaid PDL does not apply to members enrolled in a Managed Care Organization (MCO).
On October 20, 2016, the DMAS Pharmacy and Therapeutics (P&T) Committee conducted its annual review of the PDL Phase I drug classes and evaluated several new classes for addition to the PDL.
Of particular note, the P&T Committee approved criteria changes for the Hepatitis C Drug Class for the Medicaid Fee-for-Service program effective on January 1, 2017. The Committee eliminated the fibrosis scoring (Metavir) requirement as a part of the approval process and requires the prescriber to provide documentation of a Hepatitis C Patient Agreement (see attached sample Patient Agreement). These changes will also be implemented by the Medicaid health plans in the Medallion 3.0 Managed Care program effective on January 1, 2017.
The Committee approved the following changes and additions to Virginia Medicaid’s PDL:
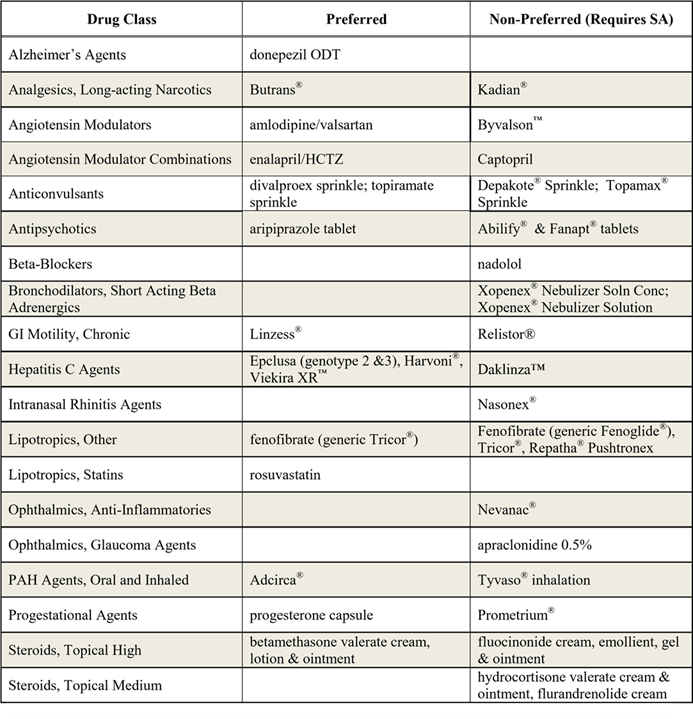
The P&T Committee approved new or revised clinical edits for several drug classes or drugs on the PDL. Clinical edit criteria for all drugs and drugs classes are detailed on the PDL. This list can be accessed at www.virginiamedicaidpharmacyservices.com/.
The P&T Committee also approved clinical edits for the following drugs:
- Aptensio® XR (stimulants)
- Naplazid® (atypical antipsychotic)
- Repatha™ (lipotropics)
- Sernivo (topical steroids)
- Taltz (cytokine and CAM antagonists)
- Vopac MDS (topical NSAIDs)
- Xrylis Kit (topical NSAIDs)
- Zinbryta (multiple sclerosis)
Virginia’s PDL can be found at http://www.dmas.virginia.gov/Content_pgs/pharm-pdl.aspx or https://www.virginiamedicaidpharmacyservices.com/. In addition a copy of the PDL can be obtained by contacting the Magellan Clinical Call Center at 1-800-932-6648. Additional information and Provider Manual updates will be posted as necessary. Comments and questions regarding this program may be emailed to pdlinput@dmas.virginia.gov.
PDL and DUR Service Authorization (SA) Process
A message indicating that a drug requires a SA will be returned at the point of sale (POS) when a prescription for a non-preferred drug is entered at point-of-sale (POS). Pharmacists should contact the member’s prescribing provider to request that they initiate the SA process. Prescribers can request a SA by letter, faxing to 1-800-932-6651, contacting the Magellan Clinical Call Center at 1-800-9326648 (available 24 hours a day, seven days a week), or by using the web-based service authorization process (Web SA). Faxed and mailed SA requests will receive a response within 24 hours of receipt. SA requests can be mailed to:
Magellan Medicaid Administration
ATTN: MAP Department/ VA Medicaid
11013 W. Broad Street, Suite 500
Glen Allen, Virginia 23060
Service authorizations forms are available online at www.virginiamedicaidpharmacyservices.com. The PDL criteria for SA purposes are also available on the same website.
PDL 72-Hour-Supply Processing Policy and Dispensing Fee Process
The PDL program provides a process where the pharmacist may dispense a 72-hour supply of a nonpreferred, prescribed medication if the prescriber is not available to consult with the pharmacist (afterhours, weekends, or holidays), AND the pharmacist, in his/her professional judgment, consistent with current standards of practice, feels that the patient’s health would be compromised without the benefit of the drug. A phone call by the pharmacy provider to Magellan Medicaid Administration at 1-800932-6648 (available 24 hours a day, seven days a week) is required for processing a 72-hour supply. The member will be charged a co-payment applicable for this 72-hour supply (partial fill). However, a co-payment will not be charged for the completion fill. The prescription must be processed as a “partial” fill and then a “completion” fill. For unit-of-use drugs (i.e., inhalers, drops, etc.), the entire unit should be dispensed and appropriate action taken to prevent similar situations in the future.
Pharmacy providers are entitled to an additional $3.75 dispensing fee when filling the completion of a 72-hour-supply prescription for a non-preferred drug. To receive the additional dispensing fee, the pharmacist must submit the 72-hour supply as a partial fill and, when submitting the claim for the completion fill, enter “03” in the “Level of Service” (data element 418-DI) field. The additional dispensing fee is only available (one time per prescription) to the pharmacist after dispensing the completion fill of a non-preferred drug when a partial (72-hour supply) prescription was previously filled.
DMAS Contact Information for Participating Pharmacies:
| Provider Information | Telephone Number(s) | Information Provided | |
| Pharmacy Call Center | 1-800-774-8481 | Pharmacy claims processing questions, including transmission errors, claims reversals, etc., the generic drug program, problems associated with generic drugs priced as brand drugs, obsolete date issues, determination if drug is eligible for Federal rebate | |
| Preferred Drug List (PDL) & | 1-800-932-6648 | Questions regarding the PDL program, service authorization requests for nonpreferred drugs, service authorization requests for drugs subject to prospective DUR edits | |
| Service Authorization Call | |||
| Center | |||
| Maximum Allowable Cost | 1-866-312-8467 | Billing disputes and general information regarding multi-source drugs subject to the MAC program. Billing disputes and general | |
| (MAC) and | information related to specialty drugs subject | ||
| Specialty Maximum | to the SMAC Program | ||
| Allowable Cost (SMAC) Call | |||
| Center | |||
| Provider Helpline | 1-800-552-8627 (in state) | All other questions concerning general Medicaid policies and procedures | |
| 1-804-786-6273 (out of state) | |||
| MediCall | 1-800-884-9730 or | Automated Voice Response System for Verifying Medicaid Eligibility | |
| 1-800-772-9996 | |||
| Medicaid Managed Care | Anthem | 1-800-901-0020 | Questions relating to Medicaid members enrolled in Medicaid Managed Care Plans |
| Organization (MCO) | Aetna | 1-800-279-1878 | |
| Information | Kaiser | 1- 855-249-5025 | |
| INTotal | 1-855-323-5588 | ||
| Optima | 1-800-881-2166 | ||
| VA Premier | 1-800-828-7989 | ||
COMMONWEALTH COORDINATED CARE
Commonwealth Coordinated Care (CCC) is a new initiative to coordinate care for individuals who are currently served by both Medicare and Medicaid and meet certain eligibility requirements. Please visit the website at http://www.dmas.virginia.gov/Content_pgs/altc-home.aspx to learn more.
MANAGED CARE ORGANIZATIONS
Many Medicaid recipients are enrolled with one of the Department’s contracted Managed Care Organizations (MCO). In order to be reimbursed for services provided to an MCO enrolled individual, providers must follow their respective contract with the MCO. The MCO may utilize different prior authorization, billing, and reimbursement guidelines than those described for Medicaid fee-for-service individuals. For more information, please contact the MCO directly. Additional information about the Medicaid MCO program can be found at http://www.dmas.virginia.gov/Content_pgs/mc-home.aspx.
VIRGINIA MEDICAID WEB PORTAL
DMAS offers a web-based Internet option to access information regarding Medicaid or FAMIS member eligibility, claims status, payment status, service limits, service authorizations, and electronic copies of remittance advices. Providers must register through the Virginia Medicaid Web Portal in order to access this information. The Virginia Medicaid Web Portal can be accessed by going to: www.virginiamedicaid.dmas.virginia.gov. If you have any questions regarding the Virginia Medicaid Web Portal, please contact the Xerox State Healthcare Web Portal Support Helpdesk, toll free, at 1866-352-0496 from 8:00 a.m. to 5:00 p.m. Monday through Friday, except holidays. The MediCall audio response system provides similar information and can be accessed by calling 1-800-884-9730 or 1-800-772-9996. Both options are available at no cost to the provider. Providers may also access service authorization information including status via KEPRO’s Provider Portal at http://dmas.kepro.com.
“HELPLINE”
The “HELPLINE” is available to answer questions Monday through Friday from 8:00 a.m. to 5:00 p.m., except on holidays. The “HELPLINE” numbers are:
1-804-786-6273 Richmond area and out-of-state long distance
1-800-552-8627 All other areas (in-state, toll-free long distance)
Please remember that the “HELPLINE” is for provider use only. Please have your Medicaid Provider Identification Number available when you call.
Hepatitis C Therapy Patient Treatment Agreement
Virginia Medicaid
Prescriber Instructions: Please submit the completed agreement with the initial prior authorization requests.
Patient Instructions: By reading and signing this agreement, I acknowledge that I have been informed about the requirements of the treatment program and understand what is expected of me. I can refuse to sign this agreement, but treatment will not be started until and unless I sign this agreement.
|
Patient Information |
Prescriber Information |
|
Name: |
Name: |
|
Medicaid Member ID Number:
|
Medicaid Provider ID Number or NPI: |
|
Date of Birth: |
Office Contact Name: |
|
Hepatitis C Medication Regimen:
|
Telephone Number: Fax Number: |
|
1. I have been told how to take my hepatitis C medicines. I understand how to take them. I am aware of possible side effects. I understand why it is important to finish all the therapy. |
|
|
2. I will take my hepatitis C medicines like my doctor said. I understand that missing doses of medicine may cause the treatment to fail. |
|
|
3. I understand that if I miss more than 3 doses in one month, Medicaid may no longer pay for my hepatitis C medicines. |
|
|
4. I will tell my doctor and pharmacist the medicines I take. I understand there may be some medicines I cannot take with my hepatitis C medicines. |
|
|
5. I understand that Medicaid may only pay for hepatitis C medicines for a certain number weeks over my lifetime. |
|
|
6. I understand that past use of certain hepatitis C medicines may keep me from using medicines like them again. |
|
|
7. I am not currently using IV drugs or abusing alcohol. |
|
|
8. I will not use IV drugs or abuse alcohol (which could seriously damage my liver) while on treatment or after completion of treatment. |
|
|
9. I am (OR my female partner is) not pregnant. |
|
|
10. I am (OR my female partner is) not planning on getting pregnant while I am on my hepatitis C medicines and for at least 6 months after I finish them. |
|
|
11. I (OR my female partner) will use two forms of non-hormonal birth control while I am taking my hepatitis C medicines and for at least 6 months after I finish taking them. |
|
|
12. I (OR my female partner) will have monthly pregnancy testing while I am taking my hepatitis C medicines. |
|
I have read the above statements and understand the agreement.
Patient Signature: _______________________________________ Date: _______________________
Physician Signature: _____________________________________ Date: _______________________

